-40%
Stainless Steel Cable Ties Guns Pliers Zip Tie Pliers Tightener Bundle
$ 22.61
- Description
- Size Guide
Description
NoteMonitors are not calibrated same, item color displayed in photos may be showing slightly different from the real object. Please take the real one as standard.
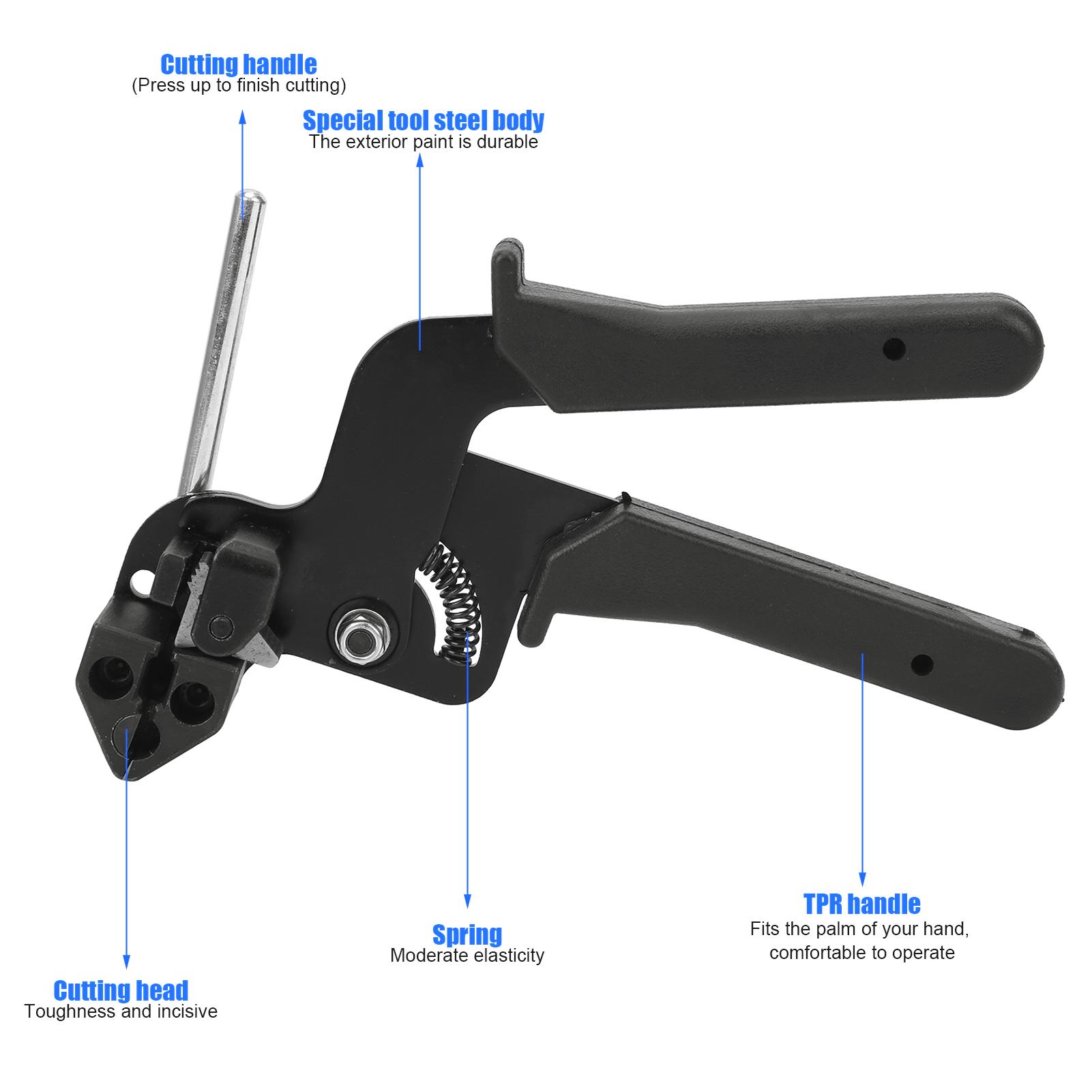
Specification
Item Type: Cable Ties Pliers
Main Material: Stainless Steel
Blade Material: 65 Manganese Steel
Handle material: TPR
Color: Black
Working Temperature: -80℃~+538℃
Tensile Strength: 100lbs
Features
1、Ergonomic handle and trigger design of cable tie tool, and you can cut the zip tie smoothly.
2、Suitable for cutting cable ties that thickness up to 0.3mm / 0.01in, width up to 12mm / 0.47in.
3、Suitable for self?locking and stepped stainless steel cable ties, such as bead?type self?locking and format self?locking.
4、Cut stainless steel cable ties, clean and without incisive edges, saving time and effort.
5、Made of premium material for anti?aging and anti?corrosion, light weight and easy to carry.
PackageList
1 x Cable Ties Pliers
FDA declaration :
Statement:The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item
This item has been cleaned and treated according to the manufacturer's instructions.
The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods(ARTG) with the code 136606. div >
The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number:K132989
The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number:K180353
The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number:K141973
massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number:K161892